INTRODUCTION When a woman experiences her period or menstruates, the menstrual cycle begins. A woman's…
Relief for Pfizer as IPAB stays Revocation on Drug Tolterodine
In a positive development for US drug giant Pfizer, the country’s Intellectual Property Appellate Board (IPAB) has issued an interim stay on an order stated by the Indian Patent Office removing a patent of Pfizer, for its extended-release drug Tolterodine (Detrol), which is used for treating old age patients who suffer from frequent urination. On the post-grant opposition by Indian pharmaceutical company Daiichi Sankyo owned Ranbaxy Laboratories, the Assistant Controller of Patents and Designs had revoked the patent in November 2013 under section 25(2)b, 25(2)c, 25(2), and 25(2)e of the Patent Act, and subsequently removed it from the registry in December last year.
Background:
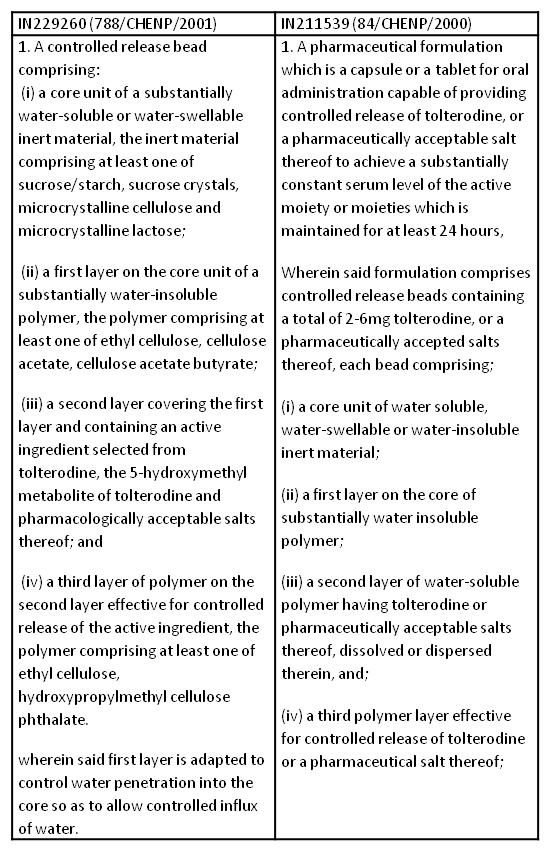
The turf war between the two multinationals started when the Chennai Patent Office revoked Pfizer’s claims on a formulation of its best-selling drug Tolterodine(Detrol). The patent on the drug was challenged by Ranbaxy Laboratories. In India, Pfizer has two patents on Tolterodine (Detrol), the first patent IN211539 was filed in August 1999, and the second (IN229260) three months later in November which cover its extended-release capsule formulation. It is believed that the invention claimed in the revoked patent (IN 229260 ) was found to be “prior claimed” by another patent of Pfizer (IN 211539 ) on the same drug under Section 25(2)(c) of prior claiming. A comparison of claims of the two patents is given below.
The invention claimed in the revoked patent was also found to be obvious, and not involving any technical advancement compared to existing knowledge under Section 25(2)(e) of obvious over the prior art. Ranbaxy relied on three prior documents i.e.; US 4800084, WO 98/03067, AND WO 96/12477. US 4800084 discloses the use of seal coat between the core and the drug layer in a multilayer medicated formulation whereas, WO 98/03067 discloses a method of use of Tolterodine in urinary voiding disorder in which various possible formulations of Tolterodine including the controlled release formulation are disclosed, but does not discuss any formulation in detail.WO96/12477 discloses a controlled release oral delivery system for oxybutynin, comprising a bead system of core and coatings. Oxybutynin is a tertiary amine antimuscarinic used to treat urinary incontinence. The controller said that the “person skilled in the art would have been motivated to prepare controlled release bead for tolterodine with the teachings of preceding prior art together with common general knowledge in the art at that time of filing the application without undue experimentation”. Further, the study carried out by the patentee on the ‘effect of seal coat thickness’ does not possess any inventive merit. The Controller further held that it is common general knowledge in the art when the thickness of the layer increases, the release rate of the drug or permeability of water decreases. The workable range of seal coat layer neither suggested nor described in the complete specification. Therefore, the present patent does not involve any technical advancement as compared to the existing knowledge. However, the opposition board sided with the patentee and opined that inventive skill is involved in selecting the quantity of the drug, core medium, and compatible polymers for the coating to arrive at a controlled release formulation as claimed in the instant patent IN 229260. The Assistant Controller revoked the said patent (IN 229260) of Tolterodine (Detrol) stating its obviousness and that it was prior claimed by another patent (IN211539) of Pfizer.
Further Developments:
Aggrieved by the developments, Pfizer moved to IPAB, requested to stay on the controller of the patent’s order. However, when the matter came up at IPAB in March 2014, Ranbaxy’s counsel submitted that they have not been given enough time by Pfizer to prepare the counter against the appeal and the matter be adjourned, which was refuted by the latter. Considering the petition, IPAB Chairman held: “The balance of convenience is very much favorable to Pfizer and accordingly we are granting an interim stay on the assistant controller’s impugned order of November 27, 2013, which revoked the patent of Pfizer.” The order was on a miscellaneous petition of Pfizer for an interim stay. The IPAB bench also observed that Ranbaxy is open to file a counter-affidavit, seeking redressal of grievances under the applicable laws. Further in March 2014, the counsel for Pfizer, PS Raman, submitted that the controller of patents had removed the patent from the register on December 15, 2013, hardly a month after the pronouncement of the revocation order in November 27, 2013. He pointed out this was done even before the limitation period for referring an appeal had expired. According to him, Pfizer had time till February 28, 2014, to appeal against the impugned order. While Pfizer informed the Board that it has served the notice to Ranbaxy on the hearing of the miscellaneous petition through courier-post on March 13, 2014, the latter in a letter dated March 21, 2014, stated that they have received the notice from the registry only on March 19, and sought a relief of adjournment. IPAB held that Ranbaxy having received the notice from the petitioner as early as March 13, 2014, had enough time to appear and argue the matter of stay petition. Hence there should not be any justification for seeking further time.
Conclusion:
Since 1991, Pfizer has been using the subject patent at the international level and filed for an Indian patent in 2001, and also seen that Pfizer did succeed in pre-grant opposition. In my opinion, this revocation decision has only reduced the Tolterodine patent term by three months ( IN 229260 expires on November 2019) since the other patent IN211539 is still valid till August 2019, but since both patents covering Tolterodine claim the same invention and one of the patents is revoked as being obvious over the prior art, the second patent is now susceptible to revocation under same grounds of obviousness and lack of inventive step and hence will be an inspiration for other competitors as well to attack the first patent.
About the Author: Sugandhika Mehta, Patent Intern at Khurana and Khurana and can be reached at [email protected]