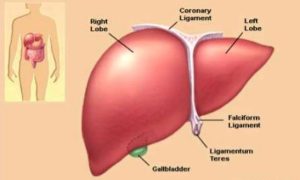
HIPATONE
HIPATONE stands out as a revolutionary solution in the landscape of liver treatment for several compelling reasons. It harnesses the power of nature through a meticulous blend of extracts from Curcuma longa, Phyllanthus emblica, and Gymnosporea montana, each renowned for their hepatoprotective and medicinal properties. Not only does it address the symptoms of liver dysfunction like jaundice, but it also targets the underlying causes with its potent anti-inflammatory and antioxidant properties. By reducing liver inflammation and protecting liver cells from oxidative damage, Hipatone not only alleviates current symptoms but also prevents further deterioration of liver function.
For more details about technology: Click Here
Patent Application No.: 153/MUM/2011 (NBA Approval awaited)
US Patent No.: US8431167B2
CARDIMODE
CARDIMODE stands out as a beacon of hope in the realm of cardiovascular health, offering a compelling solution backed by science and nature. With its unique blend of botanical ingredients, including Terminalia arjuna, Camellia sinensis, and Trikatu, this proprietary herbal medicine presents a promising avenue for individuals seeking to manage their cholesterol levels and safeguard their heart health. Through its targeted action on LDL (bad) cholesterol reduction and HDL (good) cholesterol elevation, this herbal supplement offers a holistic approach to cholesterol management, addressing a key risk factor for heart disease.
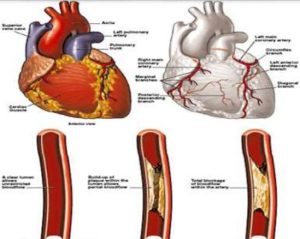
LITERATURE: UNDERSTANDING HEART ATTACK
A heart attack, or myocardial infarction, occurs when a blood vessel supplying the heart muscle is suddenly blocked by a blood clot. This blockage typically arises from the accumulation of cholesterol and other fatty substances within the blood vessel, forming plaque. Over time, plaque buildup narrows and hardens the arteries, a condition known as atherosclerosis. Reduced blood flow to the heart can lead to symptoms such as chest pain (angina) and, if left untreated, permanent damageto the heart muscle.
For more details about technology: Click Here
Patent Application Number: WO2013021302A
Masktag: Accessory Device For Face Coverings
Accessory Device for Face Coverings
Problem Statement:
The widespread use of face coverings and masks in response to health emergencies, such as the COVID-19 pandemic, has highlighted several challenges and shortcomings associated with these protective devices. Some of the key issues include:
1. Difficulty in recognizing one’s mask after temporary removal, which poses a risk of using a contaminated mask.
2. Impaired recognizability of the wearer due to the partial coverage of the face by the mask, particularly exacerbated when wearing additional accessories like glasses or hats.
3. Accumulation of unpleasant odors inside the face covering, primarily caused by the user’s breath.
4. Build-up of microbial and viral load within the face covering due to prolonged use and favorable microclimatic conditions, potentially leading to health risks.
Summary of the Invention:
The invention aims to address these challenges by providing an accessory device with dual functionality:
1. Diffuser Functionality: The device serves as a diffuser for fragrances, scents, and essential oils. This feature helps mask unpleasant odors inside the face covering and may also include antibacterial and antiviral ingredients to reduce microbial and viral load.
2. Identification Signal: The device functions as a recognition signal for the face covering or mask, enabling users to easily identify their own mask even after temporary removal. This helps prevent the accidental use of contaminated masks.
Key Features:
Reusability: The device is designed to be reusable, allowing it to be transferred to a new face covering or mask once the previous one is exhausted.
Comfort and Effectiveness: The device is intended to be comfortable to wear and effective in eliminating unpleasant odors, without causing discomfort during use.
Kit Option: The invention may also be offered as a kit, including at least one face covering or mask and at least one accessory device with diffuser and identification functionality.
Patent Application Number WO: 2022/018583
Patent Application Number India: 202317006763
For more details about technology: Click Here
Patent Protected Modular Urinal for Bed Ridden People
About Us
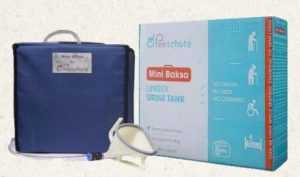
Peeschute’s modular urinal tech has been carefully designed considering factors like non-invasiveness, ease of use, hygiene, sustainability, reduced dependability and reduced risk of infections.
- Established in 2018, Peeschute is a renowned brand specializing in developing innovative solutions for sanitation.
- Peeschute has developed urinal products for travel, healthcare/homecare and outdoor spaces.
- Peeschute’s flagship product includes pocket sized disposable urine bags which solidifies up to 850 ml of urine instantly to keep it leak proof, odorless and hygienic.
- Peeschute’s products has been accolated with national and international attention including media, organizational collaborations with government and non-government bodies.
Patent Application No: 202221026004
Registrations Design Number: 373040-001 and 373041-001
For more details about technology: Click Here
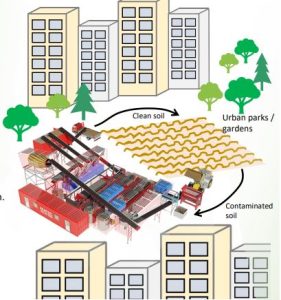
“Resoil” presented by Envit Ltd.
ReSoil® is internationally patented, groundbreaking remediation solution that enables closed-loop removal of heavy metals from contaminated soils through a zero-emission process with no wastewater, while preserving soil as a natural substrate.
 PROBLEM
PROBLEM
• Soil contamination with heavy metals is a global problem.
• Heavy metals include toxic metals such as lead (Pb), cadmium (Cd), copper (Cu) and zinc (Zn) as well as toxic metalloids such as arsenic (As) and antimony (Sb).
• Soils absorb decades of pollution from industry, intensive agriculture, traffic, waste, etc. Heavy metals are not degradable and remain in the soil.
• According to a recent conservative estimate, there are 2.8 million potentially contaminated sites in the EU (EEA, 2023).
• In China, 1/6 of the total cultivated area is contaminated with heavy metals (Yao et al., Proc Env Sci 2012, 16, 722-729).
• In New York, 71% of garden soils exceed the limits for lead and arsenic (Chenget al., Soil Sci 2015, 180, 167-174).
• Arsenic and lead are the 1st and 2nd most dangerous environmental pollutants
(ATSDR 2017).Lead distribution in surface soil of the conterminous United States (USGS 2014).
• Arsenic is highly toxic, long-term exposure can cause cancer.
• Lead is a neurotoxin; in the USA, the average lead-related loss of cognitive ability was 2.6 IQ points per person as of
2015 (McFarland et al. PNAS 2022, 119, e2118631119
For more details about technology: Click Here
Patent Application Number: WO2022184903
Machine to Dispose off used Sanitary napkins chemically
Current methods of disposal and associated danger
1. Flushing and burying
• leads to blockages, plumbing problems
• outer layer of sanitary napkin is non-degradable
• collection of napkins in heaps block the pipeline
• pollute streams and rivers
• causes hormonal changes in wildlife – enable harmful organisms in the food chain
2. Incineration
• Harmful gas emissions: dioxin, furan, nitrogen oxides, sulphur dioxide, hydrochloric acid, mercury
• Ash produced contain heavy metals like lead, cadmium, copper, zinc
• Dioxins: cancer, immune system damage, reproductive and developmental problems.
• Halogenated hydrocarbons, acid gases – impair lung function.
Objectives of the new machine
• Disposal of sanitary napkins properly, thereby wiping out the risks involved in flushing, burying, and incineration.
• Reduce pollution and health hazards of conventional methods of sanitary waste disposal.
• Reduce cost for proper disposal of sanitary napkins, tampons, baby diapers and under pads.
• Simplify the process of sanitary waste disposal.
• Help in solving greatest problem people face daily.
 Key Points of the machine
Key Points of the machine
• One machine- can be used for sanitary napkins, tampons, baby diapers and under pads. (If a method to choose the option while
using the machine is implemented)
• Can be manufactured both – domestic (for households) and industrial size (waste disposal plants/ panchayats)
Patent Application Number: 298227
For more details about technology: Click Here

 PROBLEM
PROBLEM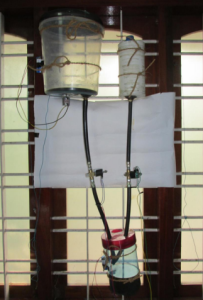 Key Points of the machine
Key Points of the machine