Introduction The Legal Dispute between Monsanto LLC and Nuziveedu Seeds Limited (NSL) changed the IP…
Ever Greening: An Abuse of the Patent System
“Drawing the line between improper attempts at evergreening and legitimate incremental innovation is a broad and difficult problem in patent law.”
In the modern world, the drive for progress and higher living standards is constant.
Science and technology are always evolving to meet these needs. However, what drives someone to work so hard to improve the lives of others?
The incentive hypothesis states that people are drawn to items that provide them with favourable incentives. Rewarding individuals for their labours will inspire others, leading to the production of higher-quality goods that will ultimately improve people’s quality of life. People in the fields of science and technology are, therefore, recognized in a variety of ways.
“One such strategy is to grant the creator of an invention the only right to ensure that no one else can profit from their labour. We refer to this right as a patent. A patent is a monopoly right given to the creator of a novel, practical object, improved new article, or novel method of producing new article.”
An innovation that is not disclosed for the benefit of humanity is entitled to a patent, which is an exclusive right that is been granted to the inventor or the assignee of the inventor for a certain amount of time by the state’s intellectual property (IP) authority. “To preserve their market supremacy, several inventive firms have taken it upon themselves to extend the patent term of their novel compounds in recent times. Pharmaceutical patenting is dominated by the monopoly term “Evergreening” extensions.”
Novartis V UOI-
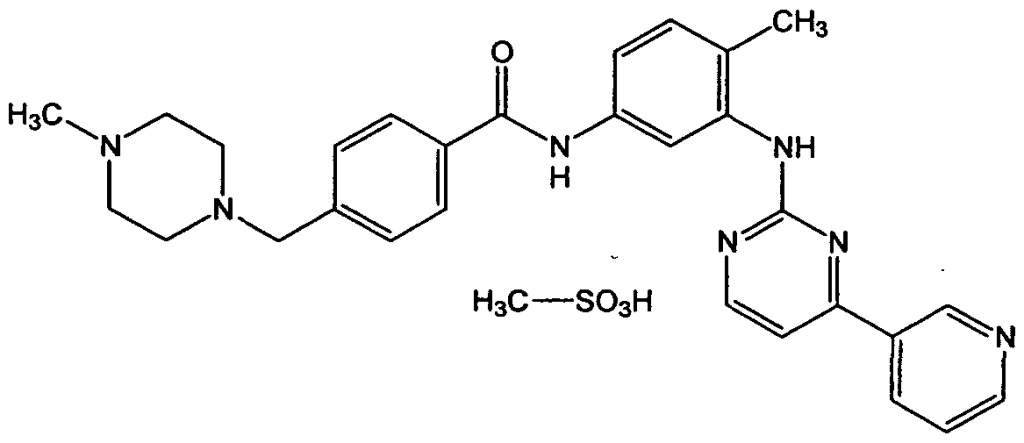
“Novartis, a Swiss company, filed a patent application in the year 1997 for Glivec, an anticancer drug used to treat chronic myeloid leukemia and gastrointestinal stromal tumors”. “The claim by the company was that they were the creators of imatinib mesylate, the 2-phenylamino-pyrimidine derivative protein’s beta crystalline salt form, which is used to treat and manage gastrointestinal stromal tumors, various cancers, including chronic myelogenous leukemia..” The drug in question was an essential one, having already received patent protection in 35 other countries.
The inventive step that was claimed by the company was that the invention was achieved by involving a two stage process wherein the company added or inserted a particular amount of beta crystalline into the basic form of imatinib. Through this, they claimed that they came out with a new invention of the existing drug as the property now was enhanced of the patented drug, and a patent must be granted for the same.
It was claimed that these qualities, which included better processing in the new form of the chemical called methane sulfonic acid, the new form so invented by the company also had better storage in addition to easy processing, this made the beta crystalline form of imatinib an inventive step and superior.
The decision of the Madras Patent office was based on “Section 3 of the Indian Patents (Amendment) Act, 1970”, where the court raised a question about the “efficacy” of the invention so claimed by the company. After deliberations and carefully examining the requirements under section 3(d), it was concluded that there was no evidence of increased effectiveness in the claimed invention.
In the end, the Court made it clear that its ruling did not indicate that incremental inventions in the fields of chemicals and pharmaceuticals would not be generally eligible for patent protection. The Court made no mention of the possibility that higher bioavailability would never lead to better therapeutic efficacy. Furthermore, it did not define “therapeutic efficacy” precisely; it may be interpreted more widely to include reduced toxicity and increased safety or, more narrowly, to include curative benefit simply.
Aims of Pharmaceuticals to use EverGreening of Patents-
“The process of evergreening a patent leads to the patent life cycle enhancement technique, which is mostly used by pharmaceutical companies to create bulletproof patent portfolios centered around profitable therapeutic compounds.” This is accomplished deftly by safeguarding many creative components of the original invention in order to prevent the impending rejection of two separate patent applications. This process so considered by various corporations leads to them having an extension of their existing patent to twenty years. Protection for this family of supposedly “lucrative molecules” can occasionally be a golden chance for global pharmaceutical companies, allowing them to maintain their market monopoly and restricting the entry of new firms capable of affecting their competition in the market. Patents cannot be extended in usual terms beyond 20 years as the regulations do not allow for such a process, so secondary patent applications are made.
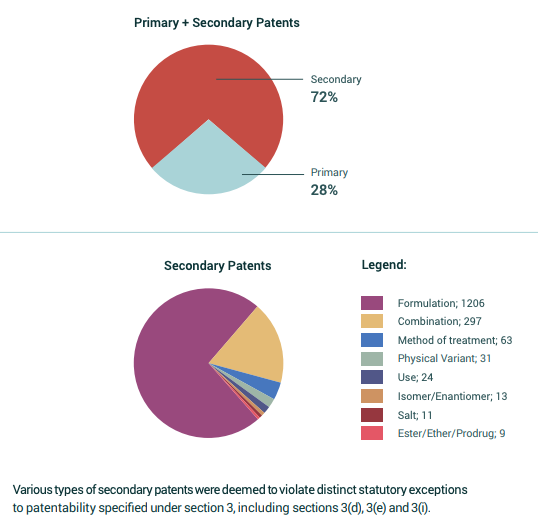
The patent office, before granting the patent of the claimed innovation, carefully scrutinizes the claims, and in order to evade such strict provisions, most companies try to shift the focus of the application from section 3(d) to 3(e) very conveniently and focus on proving the synergy.
If we talk about the pharmaceuticals industry predominantly, various stages and levels of development like manufacturing procedure, chemical composition, various formulations of compounds, processes, etc, are patentable under the respective act. I found one of the real-life examples of such a case where the firm’s aim was to get an extension of the patent term-
- As the patent for Prilosec was about to expire, AstraZeneca launched Nexium, a version of the blockbuster drug with slight design and colour changes, to maintain the drug’s monopoly.
Ever Greening is an anti-competitive practice and violates the public welfare in the perspective of consumers. The figure below explains the drug life cycle and the strategies of the pharma companies behind Ever Greening.
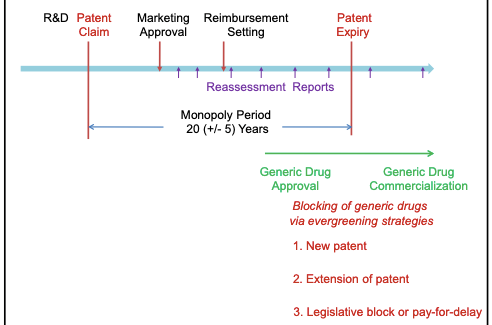
As clearly pointed out above in the blog, EverGreening gives the organization an undue advantage over its competitors as it can extend its monopoly in the market and charge higher prices from the consumer as there are no other alternatives available in the market. This is a situation that puts both consumers and competitors in a disadvantageous position. It goes against public welfare in the context of consumers and violates competition laws in India. As per the Human Rights Council and the World Medical Association, the Right to Health also constitutes one of the fundamental rights of a citizen (under Article 21 in the Constitution of India).
Author: Rakshita Ohri, in case of any queries please contact/write back to us via email to [email protected] or at Khurana & Khurana, Advocates and IP Attorney.
References
- Biotechnology Research and the Patent Paradox. (n.d.). Shodhganga.Inflibnet. Retrieved September 3, 2024, from http://shodhganga.inflibnet.ac.in:8080/jspui/bitstream/10603/26002/12/12_chapter%202.pd.
- Dwivedi, G., Hallihosur, S., & Rangan, L. (2010). Evergreening: a deceptive device in patent rights. Technology in Society, 32(4), 324-330.
- Thambisetty, S. (2014). Novartis v Union of India and the person skilled in the art: a missed opportunity. Queen Mary Journal of Intellectual Property, 4(1), 79-94.
- Kumar, A., & Nanda, A. (2017). Ever-greening in pharmaceuticals: strategies, consequences and provisions for prevention in USA, EU, India and other countries. Kumar A, Nanda A, Ever-greening in Pharmaceuticals: Strategies, Consequences and Provisions for Prevention in USA, EU, India and Other Countries. Pharm Regul Aff, 6, 185.
- Al-khafaji, A., Ravaud, P., Trinquart, L., & Desvarieux, M. Evergreening: How Green is it after all?